
Malignant
Disease:
Special Procedures
Staging
of Gynecologic
Oncology Patients With
Exploratory Laparotomy
Subclavian Port-A-Cath
Peritoneal Port-A-Cath
Application
of Vaginal
Cylinders for Intracavitary
Radiation Therapy
Application
of Uterine Afterloading Applicators
for Intracavitary Radiation Therapy
Pelvic High-Dose
Afterloader
Abdominal
Injection of Chromic Phosphate
(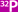 ) )
Supracolic
Total Omentectomy
Omental Pedicle "J"
Flap
Tube Gastrostomy
Total Vaginectomy
Radical
Vulvectomy
With Bilateral Inguinal
Lymph Node Dissection
Reconstruction
of the
Vulva With Gracilis Myocutaneous Flaps
Transverse
Rectus
Abdominis Myocutaneous
Flap and Vertical Rectus
Abdominis Myocutaneous
Flap
Radical
Wertheim
Hysterectomy With
Bilateral Pelvic Lymph
Node Dissection and With Extension of the Vagina
Anterior Exenteration
Posterior Exenteration
Total Pelvic
Exenteration
Colonic
"J" Pouch Rectal
Reservoir
Kock Pouch
Continent Urostomy
Omental "J" Flap
Neovagina
Ileocolic
Continent Urostomy (Miami Pouch)
Construction
of Neoanus
Gracilis Dynamic Anal
Myoplasty
Skin-Stretching
System Versus Skin Grafting
Gastric
Pelvic Flap for
Augmentation of Continent Urostomy or Neovagina
Control
of Hemorrhage in Gynecologic Surgery
Repair
of the Punctured
Vena Cava
Ligation
of a Lacerated
Internal Iliac Vein and
Suturing of a Lacerated Common Iliac Artery
Hemorrhage
Control in
Sacrospinous Ligament
Suspension of the Vagina
Presacral
Space
Hemorrhage Control
What
Not to Do in Case of Pelvic Hemorrhage
Packing
for Hemorrhage
Control
Control
of Hemorrhage
Associated With Abdominal Pregnancy |
Gastric Pelvic Flap for Augmentation
of Continent Urostomy or Neovagina
Radiation therapy is one of the keystone treatments in gynecologic
cancer. A sequela of radiation therapy, however, can be endarteritis
with fibrosis and ischemia to the pelvic tissues as well as the rectosigmoid
colon and terminal ileum. When a continent urostomy is made out of
irradiated bowel, the compliance of this radiated tissue is frequently
low; therefore, because it cannot stretch under filling with urine,
the pressure in a continent urostomy pouch will be elevated, which
may lead to incontinence.
Neovaginas made out of radiated sigmoid colon
have the same compliance features as continent urostomies. The compliance
is low, secondary to radiation fibrosis, and distensibility is minimal.
A source of highly compliant nonirradiated bowel is frequently needed
to allow a reconstructed organ such as a continent urostomy and neovagina
to have an excellent blood supply and function as desired.
The stomach is a resource available for both. It has not been irradiated.
It has copious blood supply. It secretes hydrochloric acid that reduces
urinary tract infections in the continent urostomy and provides an
acid secretion for the neovagina.
Physiologic Changes. Removal
of a small flap of gastric tissue from the greater curvature of the
stomach has few sequelae. The stomach is a highly vascular organ
and reanastomosis of the stomach heals very well. Gastrointestinal
physiology is not significantly reduced by the use of a small gastric
flap. Using the gastric flap as part of a continent urostomy changes
the physiology of the urine from alkaline to an acid, compromising
the environment for growth bacteria.
The acid secretion of a flap augmenting a sigmoid neovagina makes the
secretions more acid and allows greater distensibility through compliance
to the sigmoid neovagina.
Points of Caution. The main point of caution is the
protection of the gastroepiploic artery and the short gastric arteries
that provide the blood supply to the gastric flap.
A second point of caution is the careful removal of all staples in
the gastric flap. If present in the suture line in contact with urine,
they will be a source of stone formation.
Third, the stainless staples should never be left in a neovagina. If
present, they can cause a penile laceration.
Technique
The esophagus, spleen, and stomach
with the omentum and the right and left epigastric arteries in
place are shown. The right or left gastroepiploic arteries can
be ligated. The short gastric arteries proximal or distal to
the proposed flap are individually ligated and tied. The flap
is marked off with a skin-marking pencil. The reader is referred
to the "clam" gastrocystoplasty for the two steps involved in
stapling and cutting the flap with the gastrointestinal anastomosis
(GIA) linear stapler cutter. As seen in Figure 1 of the clam
gastrocystoplasty (Bladder and Ureter), the first GIA stapler
is placed over the drawn triangular area across the anterior
and posterior stomach wall. It is fired and cut. As seen in Figure
2 of the "clam" gastrocystoplasty, a second GIA stapler is placed
on the pencil markings of the stomach. The stapler is fired and
cut. This leaves a triangular flap of the anterior and posterior
gastric wall approximately 5-6 cm at the base and approximately
5 cm into the stomach.
Figure 3 of the clam gastrocystoplasty
shows the missing wedge-shaped flap from the greater curvature
of the stomach. Two small gastrostomies are created adjacent
to the staple line. These incisions in the stomach next to each
staple line edge of resection allow the placement of another
GIA stapler for reanastomosis. |
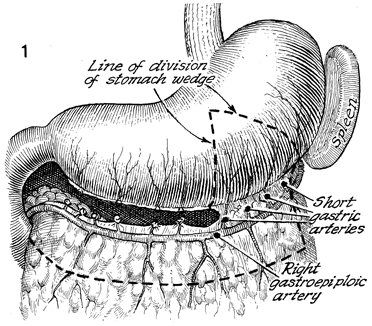
The GIA stapler is inserted into the small
gastrotomy incisions. In this figure, the GIA stapler is ghosted
as it approximates the edges of the previous staple line and,
when it is closed and activated, transects the septum created
by taking the wedge of gastric flap. In Figure 5 of the clam
gastrocystoplasty, the two gastrotomy defects are picked up with
Babcock clamps, and a TA-55 stapler is placed across these defects
in the stomach wall. Excess tissue is trimmed away. The stomach
is now continuous. All incisions have been closed with staples.
A feeding tube gastrostomy is
performed as demonstrated in Figures 6-8 of the clam gastrocystoplasty. |
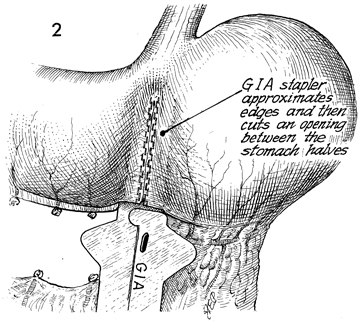
The transverse colon is retracted caudad,
and a retractor is placed under the reconstructed stomach, revealing
the vena cava (VC). The celiac artery and its branches are also
shown.
A defect is made in the mesentery
of the transverse colon medial to the middle colic vessels. The
omentum with the gastric flap attached is passed through the
defect in the mesentery of the transverse colon.
The right
gastroepiploic artery has been used in this case with the short
gastric branches attached. If the left gastroepiploic vessels
are used, the omentum and its flap are placed lateral to the
left colon. |
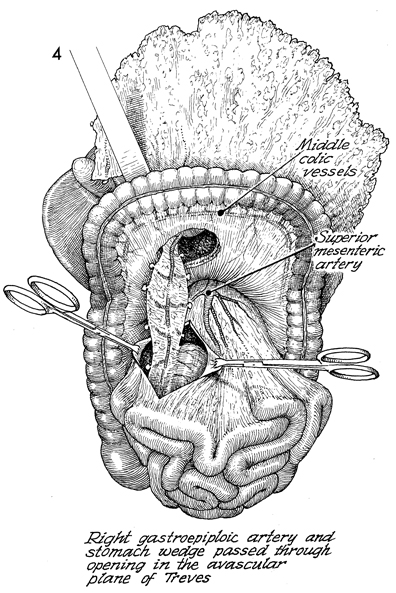
Shown here is the passage
of the omentum with its gastric flap through a defect in the
transverse colon mesentery, proceeding to a second defect created
in the avascular plane of Treves of the mesentery of the terminal
ileum. Shown also are the middle colic vessels in
the transverse colon mesentery and the superior mesenteric artery.
The right gastroepiploic artery selected in this case and the
stomach wedge-shaped flap are passed through the second opening
created in the avascular plane of Treves. This step drops the
gastric flap deep in the pelvis and makes it available for an
augmentation patch for continent urostomies or neovaginas. |
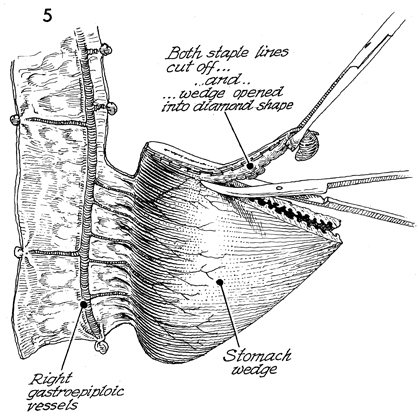
Shown here are the right gastroepiploic vessel
with its short gastric branches to the stomach and the wedge-shaped
gastric flap. Each staple is removed with sharp dissection. |
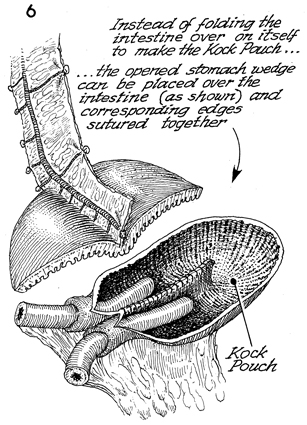
The triangular gastric flap
is now opened into a diamond-shaped flap. At the bottom, a Kock
pouch continent urostomy has been constructed out of small intestine
that may have been irradiated.
Instead of folding the intestine over
on itself to make a classic Kock pouch (see Kock Pouch Continent
Urostomy), the open diamond-shaped stomach flap can be placed over
the intestine of the Kock pouch as shown, and the corresponding
edges can be sutured with synthetic absorbable suture. |
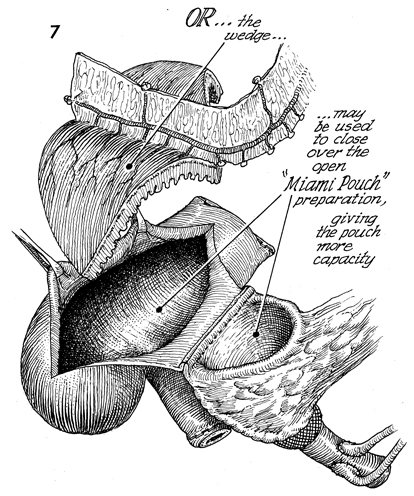
The colonic ileal pouch (Miami pouch) has
been made at the bottom. Also shown are the open right colon
and the terminal ileum, below which will become the afferent
bowel limb of the Miami pouch. At the far right, the ureters
have been implanted into a segment of ileum that has been sutured
to the medial opening of the right colon. At the top, the omental
flap with the right gastroepiploic vessels can be seen, with
the gastric flap sutured to the open colon, giving the resultant
pouch more capacity at lower pressure. |
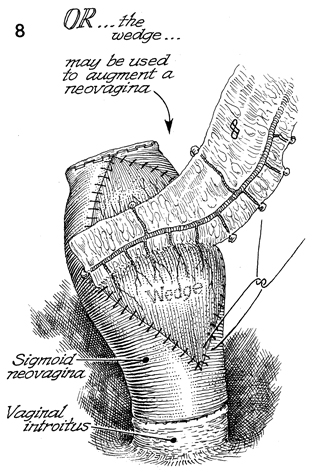
A sigmoid neovagina has been created and
sutured to the vaginal introitus. The gastric flap can be sutured
to the sigmoid neovagina in a fashion that would augment the
sigmoid neovagina that has been irradiated. This allows greater
distensibility of the vagina with improved blood supply from
a nonirradiated source. |
|








